(c) provide technical advice to the Director-General on any matter referred to it by the Director-General regarding the functioning of these Regulations.
2. The Review Committee shall be considered an expert committee and shall be subject to the WHO Advisory Panel Regulations, unless otherwise provided in this Article.
3. The Members of the Review Committee shall be selected and appointed by the Director-General from among the persons serving on the IHR Expert Roster and, when appropriate, other expert advisory panels of the Organization.
4. The Director-General shall establish the number of members to be invited to a meeting of the Review Committee, determine its date and duration, and convene the Committee.
5. The Director-General shall appoint members to the Review Committee for the duration of the work of a session only.
6. The Director-General shall select the members of the Review Committee on the basis of the principles of equitable geographical representation, gender balance, a balance of experts from developed and developing countries, representation of a diversity of scientific opinion, approaches and practical experience in various parts of the world, and an appropriate interdisciplinary balance.
1. Decisions of the Review Committee shall be taken by a majority of the members present and voting.
2. The Director-General shall invite Member States, the United Nations and its specialized agencies and other relevant intergovernmental organizations or nongovernmental organizations in official relations with WHO to designate representatives to attend the Committee sessions. Such representatives may submit memoranda and, with the consent of the Chairperson, make statements on the subjects under discussion. They shall not have the right to vote.
1. For each session, the Review Committee shall draw up a report setting forth the Committee’s views and advice. This report shall be approved by the Review Committee before the end of the session. Its views and advice shall not commit the Organization and shall be formulated as advice to the Director-General. The text of the report may not be modified without the Committee’s consent.
2. If the Review Committee is not unanimous in its findings, any member shall be entitled to express his or her dissenting professional views in an individual or group report, which shall state the reasons why a divergent opinion is held and shall form part of the Committee’s report.
3. The Review Committee’s report shall be submitted to the Director-General, who shall communicate its views and advice to the Health Assembly or the Executive Board for their consideration and action.
When the Director-General considers that a standing recommendation is necessary and appropriate for a specific public health risk, the Director-General shall seek the views of the Review Committee. In addition to the relevant paragraphs of Articles 50 to 52, the following provisions shall apply:
(a) proposals for standing recommendations, their modification or termination may be submitted to the Review Committee by the Director-General or by States Parties through the Director-General;
(b) any State Party may submit relevant information for consideration by the Review Committee;
(c) the Director-General may request any State Party, intergovernmental organization or nongovernmental organization in official relations with WHO to place at the disposal of the Review Committee information in its possession concerning the subject of the proposed standing recommendation as specified by the Review Committee;
(d) the Director-General may, at the request of the Review Committee or on the Director-General’s own initiative, appoint one or more technical experts to advise the Review Committee. They shall not have the right to vote;
(e) any report containing the views and advice of the Review Committee regarding standing recommendations shall be forwarded to the Director-General for consideration and decision. The Director-General shall communicate the Review Committee’s views and advice to the Health Assembly;
(f) the Director-General shall communicate to States Parties any standing recommendation, as well as the modifications or termination of such recommendations, together with the views of the Review Committee;
(g) standing recommendations shall be submitted by the Director-General to the subsequent Health Assembly for its consideration.
1. States Parties and the Director-General shall report to the Health Assembly on the implementation of these Regulations as decided by the Health Assembly.
2. The Health Assembly shall periodically review the functioning of these Regulations. To that end it may request the advice of the Review Committee, through the Director-General. The first such review shall take place no later than five years after the entry into force of these Regulations.
3. WHO shall periodically conduct studies to review and evaluate the functioning of Annex 2. The first such review shall commence no later than one year after the entry into force of these Regulations. The results of such reviews shall be submitted to the Health Assembly for its consideration, as appropriate.
1. Amendments to these Regulations may be proposed by any State Party or by the Director-General. Such proposals for amendments shall be submitted to the Health Assembly for its consideration.
2. The text of any proposed amendment shall be communicated to all States Parties by the Director-General at least four months before the Health Assembly at which it is proposed for consideration.
3. Amendments to these Regulations adopted by the Health Assembly pursuant to this Article shall come into force for all States Parties on the same terms, and subject to the same rights and obligations, as provided for in Article 22 of the Constitution of WHO and Articles 59 to 64 of these Regulations.
1. In the event of a dispute between two or more States Parties concerning the interpretation or application of these Regulations, the States Parties concerned shall seek in the first instance to settle the dispute through negotiation or any other peaceful means of their own choice, including good offices, mediation or conciliation. Failure to reach agreement shall not absolve the parties to the dispute from the responsibility of continuing to seek to resolve it.
2. In the event that the dispute is not settled by the means described under paragraph 1 of this Article, the States Parties concerned may agree to refer the dispute to the Director-General, who shall make every effort to settle it.
3. A State Party may at any time declare in writing to the Director-General that it accepts arbitration as compulsory with regard to all disputes concerning the interpretation or application of these Regulations to which it is a party or with regard to a specific dispute in relation to any other State Party accepting the same obligation. The arbitration shall be conducted in accordance with the Permanent Court of Arbitration Optional Rules for Arbitrating Disputes between Two States applicable at the time a request for arbitration is made. The States Parties that have agreed to accept arbitration as compulsory shall accept the arbitral award as binding and final. The Director-General shall inform the Health Assembly regarding such action as appropriate.
4. Nothing in these Regulations shall impair the rights of States Parties under any international agreement to which they may be parties to resort to the dispute settlement mechanisms of other intergovernmental organizations or established under any international agreement.
5. In the event of a dispute between WHO and one or more States Parties concerning the interpretation or application of these Regulations, the matter shall be submitted to the Health Assembly.
1. States Parties recognize that the IHR and other relevant international agreements should be interpreted so as to be compatible. The provisions of the IHR shall not affect the rights and obligations of any State Party deriving from other international agreements.
2. Subject to paragraph 1 of this Article, nothing in these Regulations shall prevent States Parties having certain interests in common owing to their health, geographical, social or economic conditions, from concluding special treaties or arrangements in order to facilitate the application of these Regulations, and in particular with regard to:
(a) the direct and rapid exchange of public health information between neighbouring territories of different States;
(b) the health measures to be applied to international coastal traffic and to international traffic in waters within their jurisdiction;
(c) the health measures to be applied in contiguous territories of different States at their common frontier;
(d) arrangements for carrying affected persons or affected human remains by means of transport specially adapted for the purpose; and
(e) deratting, disinsection, disinfection, decontamination or other treatment designed to render goods free of disease-causing agents.
3. Without prejudice to their obligations under these Regulations, States Parties that are members of a regional economic integration organization shall apply in their mutual relations the common rules in force in that regional economic integration organization.
1. These Regulations, subject to the provisions of Article 62 and the exceptions hereinafter provided, shall replace as between the States bound by these Regulations and as between these States and WHO, the provisions of the following international sanitary agreements and regulations:
(a) International Sanitary Convention, signed in Paris, 21 June 1926;
(b) International Sanitary Convention for Aerial Navigation, signed at The Hague, 12 April 1933;
(c) International Agreement for dispensing with Bills of Health, signed in Paris, 22 December 1934;
(d) International Agreement for dispensing with Consular Visas on Bills of Health, signed in Paris, 22 December 1934;
(e) Convention modifying the International Sanitary Convention of 21 June 1926, signed in Paris, 31 October 1938;
(f) International Sanitary Convention, 1944, modifying the International Sanitary Convention of 21 June 1926, opened for signature in Washington, 15 December 1944;
(g) International Sanitary Convention for Aerial Navigation, 1944, modifying the International Sanitary Convention of 12 April 1933, opened for signature in Washington, 15 December 1944;
(h) Protocol of 23 April 1946 to prolong the International Sanitary Convention, 1944, signed in Washington;
(i) Protocol of 23 April 1946 to prolong the International Sanitary Convention for Aerial Navigation, 1944, signed in Washington;
(j) International Sanitary Regulations, 1951, and the Additional Regulations of 1955, 1956, 1960, 1963 and 1965; and
(k) the International Health Regulations of 1969 and the amendments of 1973 and 1981.
2. The Pan American Sanitary Code, signed at Havana, 14 November 1924, shall remain in force with the exception of Articles 2, 9, 10, 11, 16 to 53 inclusive, 61 and 62, to which the relevant part of paragraph 1 of this Article shall apply.
1. The period provided in execution of Article 22 of the Constitution of WHO for rejection of, or reservation to, these Regulations or an amendment thereto, shall be 18 months from the date of the notification by the Director-General of the adoption of these Regulations or of an amendment to these Regulations by the Health Assembly. Any rejection or reservation received by the Director-General after the expiry of that period shall have no effect.
2. These Regulations shall enter into force 24 months after the date of notification referred to in paragraph 1 of this Article, except for:
(a) a State that has rejected these Regulations or an amendment thereto in accordance with Article 61;
(b) a State that has made a reservation, for which these Regulations shall enter into force as provided in Article 62;
(c) a State that becomes a Member of WHO after the date of the notification by the Director-General referred to in paragraph 1 of this Article, and which is not already a party to these Regulations, for which these Regulations shall enter into force as provided in Article 60; and
(d) a State not a Member of WHO that accepts these Regulations, for which they shall enter into force in accordance with paragraph 1 of Article 64.
3. If a State is not able to adjust its domestic legislative and administrative arrangements fully with these Regulations within the period set out in paragraph 2 of this Article, that State shall submit within the period specified in paragraph 1 of this Article a declaration to the Director-General regarding the outstanding adjustments and achieve them no later than 12 months after the entry into force of these Regulations for that State Party.
Any State which becomes a Member of WHO after the date of the notification by the Director-General referred to in paragraph 1 of Article 59, and which is not already a party to these Regulations, may communicate its rejection of, or any reservation to, these Regulations within a period of twelve months from the date of the notification to it by the Director-General after becoming a Member of WHO. Unless rejected, these Regulations shall enter into force with respect to that State, subject to the provisions of Articles 62 and 63, upon expiry of that period. In no case shall these Regulations enter into force in respect to that State earlier than 24 months after the date of notification referred to in paragraph 1 of Article 59.
If a State notifies the Director-General of its rejection of these Regulations or of an amendment thereto within the period provided in paragraph 1 of Article 59, these Regulations or the amendment concerned shall not enter into force with respect to that State. Any international sanitary agreement or regulations listed in Article 58 to which such State is already a party shall remain in force as far as such State is concerned.
1. States may make reservations to these Regulations in accordance with this Article. Such reservations shall not be incompatible with the object and purpose of these Regulations.
2. Reservations to these Regulations shall be notified to the Director-General in accordance with paragraph 1 of Article 59 and Article 60, paragraph 1 of Article 63 or paragraph 1 of Article 64, as the case may be. A State not a Member of WHO shall notify the Director-General of any reservation with its notification of acceptance of these Regulations. States formulating reservations should provide the Director-General with reasons for the reservations.
3. A rejection in part of these Regulations shall be considered as a reservation.
4. The Director-General shall, in accordance with paragraph 2 of Article 65, issue notification of each reservation received pursuant to paragraph 2 of this Article. The Director-General shall:
(a) if the reservation was made before the entry into force of these Regulations, request those Member States that have not rejected these Regulations to notify him or her within six months of any objection to the reservation, or
(b) if the reservation was made after the entry into force of these Regulations, request States Parties to notify him or her within six months of any objection to the reservation.
States objecting to a reservation should provide the Director-General with reasons for the objection.
5. After this period, the Director-General shall notify all States Parties of the objections he or she has received with regard to reservations. Unless by the end of six months from the date of the notification referred to in paragraph 4 of this Article a reservation has been objected to by one-third of the States referred to in paragraph 4 of this Article, it shall be deemed to be accepted and these Regulations shall enter into force for the reserving State, subject to the reservation.
6. If at least one-third of the States referred to in paragraph 4 of this Article object to the reservation by the end of six months from the date of the notification referred to in paragraph 4 of this Article, the Director-General shall notify the reserving State with a view to its considering withdrawing the reservation within three months from the date of the notification by the Director-General.
7. The reserving State shall continue to fulfil any obligations corresponding to the subject matter of the reservation, which the State has accepted under any of the international sanitary agreements or regulations listed in Article 58.
8. If the reserving State does not withdraw the reservation within three months from the date of the notification by the Director-General referred to in paragraph 6 of this Article, the Director-General shall seek the view of the Review Committee if the reserving State so requests. The Review Committee shall advise the Director-General as soon as possible and in accordance with Article 50 on the practical impact of the reservation on the operation of these Regulations.
9. The Director-General shall submit the reservation, and the views of the Review Committee if applicable, to the Health Assembly for its consideration. If the Health Assembly, by a majority vote, objects to the reservation on the ground that it is incompatible with the object and purpose of these Regulations, the reservation shall not be accepted and these Regulations shall enter into force for the reserving State only after it withdraws its reservation pursuant to Article 63. If the Health Assembly accepts the reservation, these Regulations shall enter into force for the reserving State, subject to its reservation.
1. A rejection made under Article 61 may at any time be withdrawn by a State by notifying the Director-General. In such cases, these Regulations shall enter into force with regard to that State upon receipt by the Director-General of the notification, except where the State makes a reservation when withdrawing its rejection, in which case these Regulations shall enter into force as provided in Article 62. In no case shall these Regulations enter into force in respect to that State earlier than 24 months after the date of notification referred to in paragraph 1 of Article 59.
2. The whole or part of any reservation may at any time be withdrawn by the State Party concerned by notifying the Director-General. In such cases, the withdrawal will be effective from the date of receipt by the Director-General of the notification.
1. Any State not a Member of WHO, which is a party to any international sanitary agreement or regulations listed in Article 58 or to which the Director-General has notified the adoption of these Regulations by the World Health Assembly, may become a party hereto by notifying its acceptance to the Director-General and, subject to the provisions of Article 62, such acceptance shall become effective upon the date of entry into force of these Regulations, or, if such acceptance is notified after that date, three months after the date of receipt by the Director-General of the notification of acceptance.
2. Any State not a Member of WHO which has become a party to these Regulations may at any time withdraw from participation in these Regulations, by means of a notification addressed to the Director-General which shall take effect six months after the Director-General has received it. The State which has withdrawn shall, as from that date, resume application of the provisions of any international sanitary agreement or regulations listed in Article 58 to which it was previously a party.
1. The Director-General shall notify all States Members and Associate Members of WHO, and also other parties to any international sanitary agreement or regulations listed in Article 58, of the adoption by the Health Assembly of these Regulations.
2. The Director-General shall also notify these States, as well as any other State which has become a party to these Regulations or to any amendment to these Regulations, of any notification received by WHO under Articles 60 to 64 respectively, as well as of any decision taken by the Health Assembly under Article 62.
1. The Arabic, Chinese, English, French, Russian and Spanish texts of these Regulations shall be equally authentic. The original texts of these Regulations shall be deposited with WHO.
2. The Director-General shall send, with the notification provided in paragraph 1 of Article 59, certified copies of these Regulations to all Members and Associate Members, and also to other parties to any of the international sanitary agreements or regulations listed in Article 58.
3. Upon the entry into force of these Regulations, the Director-General shall deliver certified copies thereof to the Secretary-General of the United Nations for registration in accordance with Article 102 of the Charter of the United Nations.
1. States Parties shall utilize existing national structures and resources to meet their core capacity requirements under these Regulations, including with regard to:
(a) their surveillance, reporting, notification, verification, response and collaboration activities; and
(b) their activities concerning designated airports, ports and ground crossings.
2. Each State Party shall assess, within two years following the entry into force of these Regulations for that State Party, the ability of existing national structures and resources to meet the minimum requirements described in this Annex. As a result of such assessment, States Parties shall develop and implement plans of action to ensure that these core capacities are present and functioning throughout their territories as set out in paragraph 1 of Article 5 and paragraph 1 of Article 13.
3. States Parties and WHO shall support assessments, planning and implementation processes under this Annex.
4. At the local community level and/or primary public health response level
The capacities:
(a) to detect events involving disease or death above expected levels for the particular time and place in all areas within the territory of the State Party; and
(b) to report all available essential information immediately to the appropriate level of health-care response. At the community level, reporting shall be to local community health-care institutions or the appropriate health personnel. At the primary public health response level, reporting shall be to the intermediate or national response level, depending on organizational structures. For the purposes of this Annex, essential information includes the following: clinical descriptions, laboratory results, sources and type of risk, numbers of human cases and deaths, conditions affecting the spread of the disease and the health measures employed; and
(c) to implement preliminary control measures immediately.
5. At the intermediate public health response levels
The capacities:
(a) to confirm the status of reported events and to support or implement additional control measures; and
(b) to assess reported events immediately and, if found urgent, to report all essential information to the national level. For the purposes of this Annex, the criteria for urgent events include serious public health impact and/or unusual or unexpected nature with high potential for spread.
6. At the national level
Assessment and notification. The capacities:
(a) to assess all reports of urgent events within 48 hours; and
(b) to notify WHO immediately through the National IHR Focal Point when the assessment indicates the event is notifiable pursuant to paragraph 1 of Article 6 and Annex 2 and to inform WHO as required pursuant to Article 7 and paragraph 2 of Article 9.
Public health response. The capacities:
(a) to determine rapidly the control measures required to prevent domestic and international spread;
(b) to provide support through specialized staff, laboratory analysis of samples (domestically or through collaborating centres) and logistical assistance (e.g. equipment, supplies and transport);
(c) to provide on-site assistance as required to supplement local investigations;
(d) to provide a direct operational link with senior health and other officials to approve rapidly and implement containment and control measures;
(e) to provide direct liaison with other relevant government ministries;
(f) to provide, by the most efficient means of communication available, links with hospitals, clinics, airports, ports, ground crossings, laboratories and other key operational areas for the dissemination of information and recommendations received from WHO regarding events in the State Party’s own territory and in the territories of other States Parties;
(g) to establish, operate and maintain a national public health emergency response plan, including the creation of multidisciplinary/multisectoral teams to respond to events that may constitute a public health emergency of international concern; and
(h) to provide the foregoing on a 24-hour basis.
1. At all times
The capacities:
(a) to provide access to (i) an appropriate medical service including diagnostic facilities located so as to allow the prompt assessment and care of ill travellers, and (ii) adequate staff, equipment and premises;
(b) to provide access to equipment and personnel for the transport of ill travellers to an appropriate medical facility;
(c) to provide trained personnel for the inspection of conveyances;
(d) to ensure a safe environment for travellers using point of entry facilities, including potable water supplies, eating establishments, flight catering facilities, public washrooms, appropriate solid and liquid waste disposal services and other potential risk areas, by conducting inspection programmes, as appropriate; and
(e) to provide as far as practicable a programme and trained personnel for the control of vectors and reservoirs in and near points of entry.
2. For responding to events that may constitute a public health emergency of international concern
The capacities:
(a) to provide appropriate public health emergency response by establishing and maintaining a public health emergency contingency plan, including the nomination of a coordinator and contact points for relevant point of entry, public health and other agencies and services;
(b) to provide assessment of and care for affected travellers or animals by establishing arrangements with local medical and veterinary facilities for their isolation, treatment and other support services that may be required;
(c) to provide appropriate space, separate from other travellers, to interview suspect or affected persons;
(d) to provide for the assessment and, if required, quarantine of suspect travellers, preferably in facilities away from the point of entry;
(e) to apply recommended measures to disinsect, derat, disinfect, decontaminate or otherwise treat baggage, cargo, containers, conveyances, goods or postal parcels including, when appropriate, at locations specially designated and equipped for this purpose;
(f) to apply entry or exit controls for arriving and departing travellers; and
(g) to provide access to specially designated equipment, and to trained personnel with appropriate personal protection, for the transfer of travellers who may carry infection or contamination.
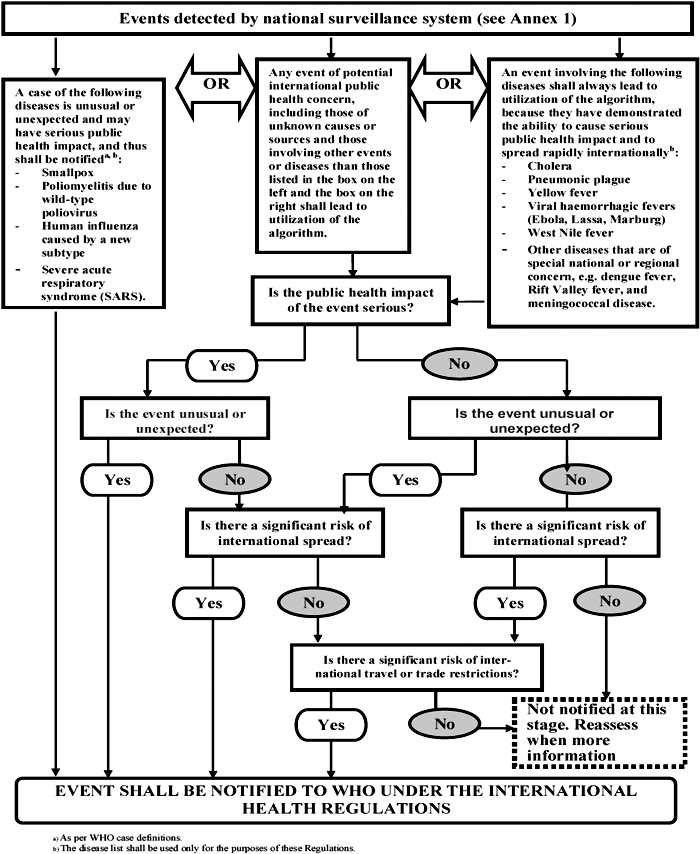
The examples appearing in this Annex are not binding and are for indicative guidance purposes to assist in the interpretation of the decision instrument criteria.
DOES THE EVENT MEET AT LEAST TWO OF THE FOLLOWING CRITERIA?
| I. Is the public health impact of the event serious? | ||
| 1. Is the number of cases and/or number of deaths for this type of event large for the given place, time or population? | ||
| 2. Has the event the potential to have a high public health impact? | ||
| THE FOLLOWING ARE EXAMPLES OF CIRCUMSTANCES THAT CONTRIBUTE TO HIGH PUBLIC HEALTH IMPACT: | ||
| - Event caused by a pathogen with high potential to cause epidemic (infectiousness of the agent, high case fatality, multiple transmission routes or healthy carrier). | ||
| - Indication of treatment failure (new or emerging antibiotic resistance, vaccine failure, antidote resistance or failure). | ||
| - Event represents a significant public health risk even if no or very few human cases have yet been identified. | ||
| Is the public health | - Cases reported among health staff. | |
| impact of the event serious? | - The population at risk is especially vulnerable (refugees, low level of immunization, children, elderly, low immunity, undernourished, etc.). | |
| - Concomitant factors that may hinder or delay the public health response (natural catastrophes, armed conflicts, unfavourable weather conditions, multiple foci in the State Party). | ||
| - Event in an area with high population density. | ||
| - Spread of toxic, infectious or otherwise hazardous materials that may be occurring naturally or otherwise that has contaminated or has the potential to contaminate a population and/or a large geographical area. | ||
| 3. Is external assistance needed to detect, investigate, respond and control the current event, or prevent new cases? | ||
| THE FOLLOWING ARE EXAMPLES OF WHEN ASSISTANCE MAY BE REQUIRED: | ||
| - Inadequate human, financial, material or technical resources - in particular: | ||
| = Insufficient laboratory or epidemiological capacity to investigate the event (equipment, personnel, financial resources) | ||
| = Insufficient antidotes, drugs and/or vaccine and/or protective equipment, decontamination equipment, or supportive equipment to cover estimated needs | ||
| = Existing surveillance system is inadequate to detect new cases in a timely manner. | ||
| IS THE PUBLIC HEALTH IMPACT OF THE EVENT SERIOUS? | ||
| Answer „yes” if you have answered „yes” to questions 1, 2 or 3 above. | ||
| II. Is the event unusual or unexpected? | ||
| 4. Is the event unusual? | ||
| THE FOLLOWING ARE EXAMPLES OF UNUSUAL EVENTS: | ||
| - The event is caused by an unknown agent or the source, vehicle, route of transmission is unusual or unknown. | ||
| Is the event unusual or unexpected | - Evolution of cases more severe than expected (including morbidity or case-fatality) or with unusual symptoms. | |
| - Occurrence of the event itself unusual for the area, season or population. | ||
| 5. Is the event unexpected from a public health perspective? | ||
| THE FOLLOWING ARE EXAMPLES OF UNEXPECTED EVENTS: | ||
| - Event caused by a disease/agent that had already been eliminated or eradicated from the State Party or not previously reported. | ||
| IS THE EVENT UNUSUAL OR UNEXPECTED? | ||
| Answer „yes” if you have answered „yes” to questions 4 or 5 above. | ||
| III. Is there a significant risk of international spread? | ||
| 6. Is there evidence of an epidemiological link to similar events in other States? | ||
| 7. Is there any factor that should alert us to the potential for cross border movement of the agent, vehicle or host? | ||
| THE FOLLOWING ARE EXAMPLES OF CIRCUMSTANCES THAT MAY PREDISPOSE TO INTERNATIONAL SPREAD: | ||
| Is there a significant risk of international | - Where there is evidence of local spread, an index case (or other linked cases) with a history within the previous month of: | |
| spread | = international travel (or time equivalent to the incubation period if the pathogen is known) | |
| = participation in an international gathering (pilgrimage, sports event, conference, etc.) | ||
| = close contact with an international traveller or a highly mobile population. | ||
| - Event caused by an environmental contamination that has the potential to spread across international borders. | ||
| - Event in an area of intense international traffic with limited capacity for sanitary control or environmental detection or decontamination. | ||
| IS THERE A SIGNIFICANT RISK OF INTERNATIONAL SPREAD? | ||
| Answer „yes” if you have answered „yes” to questions 6 or 7 above. | ||
| IV. Is there a significant risk of international travel or trade restrictions? | ||
| 8. Have similar events in the past resulted in international restriction on trade and/or travel? | ||
| 9. Is the source suspected or known to be a food product, water or any other goods that might be contaminated that has been exported/imported to/from other States? | ||
| Risk of international restrictions? | 10. Has the event occurred in association with an international gathering or in an area of intense international tourism? | |
| 11. Has the event caused requests for more information by foreign officials or international media? | ||
| IS THERE A SIGNIFICANT RISK OF INTERNATIONAL TRADE OR TRAVEL RESTRICTIONS? | ||
| Answer „yes” if you have answered „yes” to questions 8, 9, 10 or 11 above. | ||
States Parties that answer „yes” to the question whether the event meets any two of the four criteria (I-IV) above, shall notify WHO under Article 6 of the International Health Regulations.
Port of .............................. Date: ..................................
This Certificate records the inspection and 1) exemption from control or 2) control measures applied
Name of ship or inland navigation vessel ............................................. Flag .......................... Registration/IMO No. ................
At the time of inspection the holds were unladen/laden with ........... tonnes of ............................................................. cargo
Name and address of inspecting officer ...........................................
Ship Sanitation Control Exemption Certificate Ship Sanitation Control Certificate
| Areas [systems, and services] inspected | Evidence found1 | Sample results2 | Documents reviewed | Control measures applied | Re-inspection date | Comments regarding conditions found | ||
| Galley | Medical log | |||||||
| Pantry | Ship’s log | |||||||
| Stores | Other | |||||||
| Hold(s)/cargo | ||||||||
| Quarters: | ||||||||
| - crew | ||||||||
| - officers | ||||||||
| - passengers | ||||||||
| - deck | ||||||||
| Potable water | ||||||||
| Sewage | ||||||||
| Ballast tanks | ||||||||
| Solid and medical waste | ||||||||
| Standing water | ||||||||
| Engine room | ||||||||
| Medical facilities | ||||||||
| Other areas specified - see attached | ||||||||
| Note areas not applicable, by marking N/A. |
No evidence found. Ship/vessel is exempted from control measures. Control measures indicated were applied on the date below.
Name and designation of issuing officer ...................................................................... Signature and seal ................................. Date .....................
| 1 (a) Evidence of infection or contamination, including: vectors in all stages of growth; animal reservoirs for vectors; rodents or other species that could carry human disease, microbiological, chemical and other risks to human health; signs of inadequate sanitary measures. (b) Information concerning any human cases (to be included in the Maritime Declaration of Health). | ||
| 2 Results from samples taken on board. Analysis to be provided to ship’s master by most expedient means and, if re-inspection is required, to the next appropriate port of call coinciding with the re-inspection date specified in this certificate. | ||
| Sanitation Control Exemption Certificates and Sanitation Control Certificates are valid for a maximum of six months, but the validity period may be extended by one month if inspection cannot be carried out at the port and there is no evidence of infection or contamination. | ||
| Areas/facilities/systems inspected | Evidence found | Sample results | Documents reviewed | Control measures applied | Re-inspection date | Comments regarding conditions found | |
| Food | |||||||
| Source | |||||||
| Storage | |||||||
| Preparation | |||||||
| Service | |||||||
| Water | |||||||
| Source | |||||||
| Storage | |||||||
| Distribution | |||||||
| Waste | |||||||
| Holding | |||||||
| Treatment | |||||||
| Disposal | |||||||
| Swimming pools/spas | |||||||
| Equipment | |||||||
| Operation | |||||||
| Medical facilities | |||||||
| Equipment and medical devices | |||||||
| Operation | |||||||
| Medicines | |||||||
| Other areas inspected | |||||||
Indicate when the areas listed are not applicable by marking N/A.
1. Conveyance operators shall facilitate:
(a) inspections of the cargo, containers and conveyance;
(b) medical examinations of persons on board;
(c) application of other health measures under these Regulations; and
(d) provision of relevant public health information requested by the State Party.
2. Conveyance operators shall provide to the competent authority a valid Ship Sanitation Control Exemption Certificate or a Ship Sanitation Control Certificate or a Maritime Declaration of Health, or the Health Part of an Aircraft General Declaration, as required under these Regulations.
1. Control measures applied to baggage, cargo, containers, conveyances and goods under these Regulations shall be carried out so as to avoid as far as possible injury or discomfort to persons or damage to the baggage, cargo, containers, conveyances and goods. Whenever possible and appropriate, control measures shall be applied when the conveyance and holds are empty.
2. States Parties shall indicate in writing the measures applied to cargo, containers or conveyances, the parts treated, the methods employed, and the reasons for their application. This information shall be provided in writing to the person in charge of an aircraft and, in case of a ship, on the Ship Sanitation Control Certificate. For other cargo, containers or conveyances, States Parties shall issue such information in writing to consignors, consignees, carriers, the person in charge of the conveyance or their respective agents.
1. WHO shall publish, on a regular basis, a list of areas where disinsection or other vector control measures are recommended for conveyances arriving from these areas. Determination of such areas shall be made pursuant to the procedures regarding temporary or standing recommendations, as appropriate.
2. Every conveyance leaving a point of entry situated in an area where vector control is recommended should be disinsected and kept free of vectors. When there are methods and materials advised by the Organization for these procedures, these should be employed. The presence of vectors on board conveyances and the control measures used to eradicate them shall be included:
(a) in the case of aircraft, in the Health Part of the Aircraft General Declaration, unless this part of the Declaration is waived by the competent authority at the airport of arrival;
(b) in the case of ships, on the Ship Sanitation Control Certificates; and
(c) in the case of other conveyances, on a written proof of treatment issued to the consignor, consignee, carrier, the person in charge of the conveyance or their agent, respectively.
3. States Parties should accept disinsecting, deratting and other control measures for conveyances applied by other States if methods and materials advised by the Organization have been applied.
4. States Parties shall establish programmes to control vectors that may transport an infectious agent that constitutes a public health risk to a minimum distance of 400 metres from those areas of point of entry facilities that are used for operations involving travellers, conveyances, containers, cargo and postal parcels, with extension of the minimum distance if vectors with a greater range are present.
5. If a follow-up inspection is required to determine the success of the vector control measures applied, the competent authorities for the next known port or airport of call with a capacity to make such an inspection shall be informed of this requirement in advance by the competent authority advising such follow-up. In the case of ships, this shall be noted on the Ship Sanitation Control Certificate.
6. A conveyance may be regarded as suspect and should be inspected for vectors and reservoirs if:
(a) it has a possible case of vector-borne disease on board;
(b) a possible case of vector-borne disease has occurred on board during an international voyage; or
(c) it has left an affected area within a period of time where on-board vectors could still carry disease.
7. A State Party should not prohibit the landing of an aircraft or berthing of a ship in its territory if the control measures provided for in paragraph 3 of this Annex or otherwise recommended by the Organization are applied. However, aircraft or ships coming from an affected area may be required to land at airports or divert to another port specified by the State Party for that purpose.
8. A State Party may apply vector control measures to a conveyance arriving from an area affected by a vector-borne disease if the vectors for the foregoing disease are present in its territory.
1. Vaccines or other prophylaxis specified in Annex 7 or recommended under these Regulations shall be of suitable quality; those vaccines and prophylaxis designated by WHO shall be subject to its approval. Upon request, the State Party shall provide to WHO appropriate evidence of the suitability of vaccines and prophylaxis administered within its territory under these Regulations.
2. Persons undergoing vaccination or other prophylaxis under these Regulations shall be provided with an international certificate of vaccination or prophylaxis (hereinafter the „certificate”) in the form specified in this Annex. No departure shall be made from the model of the certificate specified in this Annex.
3. Certificates under this Annex are valid only if the vaccine or prophylaxis used has been approved by WHO.
4. Certificates must be signed in the hand of the clinician, who shall be a medical practitioner or other authorized health worker, supervising the administration of the vaccine or prophylaxis. The certificate must also bear the official stamp of the administering centre; however, this shall not be an accepted substitute for the signature.
5. Certificates shall be fully completed in English or in French. They may also be completed in another language, in addition to either English or French.
6. Any amendment of this certificate, or erasure, or failure to complete any part of it, may render it invalid.
7. Certificates are individual and shall in no circumstances be used collectively. Separate certificates shall be issued for children.
8. A parent or guardian shall sign the certificate when the child is unable to write. The signature of an illiterate shall be indicated in the usual manner by the person’s mark and the indication by another that this is the mark of the person concerned.
9. If the supervising clinician is of the opinion that the vaccination or prophylaxis is contraindicated on medical grounds, the supervising clinician shall provide the person with reasons, written in English or French, and where appropriate in another language in addition to English or French, underlying that opinion, which the competent authorities on arrival should take into account. The supervising clinician and competent authorities shall inform such persons of any risk associated with non-vaccination and with the non-use of prophylaxis in accordance with paragraph 4 of Article 23.
10. An equivalent document issued by the Armed Forces to an active member of those Forces shall be accepted in lieu of an international certificate in the form shown in this Annex if:
(a) it embodies medical information substantially the same as that required by such form; and
(b) it contains a statement in English or in French and where appropriate in another language in addition to English or French recording the nature and date of the vaccination or prophylaxis and to the effect that it is issued in accordance with this paragraph.
MODEL INTERNATIONAL CERTIFICATE OF VACCINATION OR PROPHYLAXIS
This is to certify that [name] ............................, date of birth ........................, sex ................., nationality ......................................, national identification document, if applicable .................. whose signature follows ..........................................................................
has on the date indicated been vaccinated or received prophylaxis against:
(name of disease or condition) ..............................................................................
in accordance with the International Health Regulations.
| Vaccine or prophylaxis | Date | Signature and professional status of supervising clinician | Manufacturer and batch No. of vaccine or prophylaxis | Certificate valid from ....... until ............ | Official stamp of administering centre | |
| 1. | ||||||
| 2. |
This certificate is valid only if the vaccine or prophylaxis used has been approved by the World Health Organization.
This certificate must be signed in the hand of the clinician, who shall be a medical practitioner or other authorized health worker, supervising the administration of the vaccine or prophylaxis. The certificate must also bear the official stamp of the administering centre; however, this shall not be an accepted substitute for the signature.
Any amendment of this certificate, or erasure, or failure to complete any part of it, may render it invalid.
The validity of this certificate shall extend until the date indicated for the particular vaccination or prophylaxis. The certificate shall be fully completed in English or in French. The certificate may also be completed in another language on the same document, in addition to either English or French.
1. In addition to any recommendation concerning vaccination or prophylaxis, the following diseases are those specifically designated under these Regulations for which proof of vaccination or prophylaxis may be required for travellers as a condition of entry to a State Party:
Vaccination against yellow fever.
2. Recommendations and requirements for vaccination against yellow fever:
(a) For the purpose of this Annex:
(i) the incubation period of yellow fever is six days;
(ii) yellow fever vaccines approved by WHO provide protection against infection starting 10 days following the administration of the vaccine;
(iii) this protection continues for 10 years; and
(iv) the validity of a certificate of vaccination against yellow fever shall extend for a period of 10 years, beginning 10 days after the date of vaccination or, in the case of a revaccination within such period of 10 years, from the date of that revaccination.
(b) Vaccination against yellow fever may be required of any traveller leaving an area where the Organization has determined that a risk of yellow fever transmission is present.
(c) If a traveller is in possession of a certificate of vaccination against yellow fever which is not yet valid, the traveller may be permitted to depart, but the provisions of paragraph 2(h) of this Annex may be applied on arrival.
(d) A traveller in possession of a valid certificate of vaccination against yellow fever shall not be treated as suspect, even if coming from an area where the Organization has determined that a risk of yellow fever transmission is present.
(e) In accordance with paragraph 1 of Annex 6 the yellow fever vaccine used must be approved by the Organization.
(f) States Parties shall designate specific yellow fever vaccination centres within their territories in order to ensure the quality and safety of the procedures and materials employed.
(g) Every person employed at a point of entry in an area where the Organization has determined that a risk of yellow fever transmission is present, and every member of the crew of a conveyance using any such point of entry, shall be in possession of a valid certificate of vaccination against yellow fever.
(h) A State Party, in whose territory vectors of yellow fever are present, may require a traveller from an area where the Organization has determined that a risk of yellow fever transmission is present, who is unable to produce a valid certificate of vaccination against yellow fever, to be quarantined until the certificate becomes valid, or until a period of not more than six days, reckoned from the date of last possible exposure to infection, has elapsed, whichever occurs first.
(i) Travellers who possess an exemption from yellow fever vaccination, signed by an authorized medical officer or an authorized health worker, may nevertheless be allowed entry, subject to the provisions of the foregoing paragraph of this Annex and to being provided with information regarding protection from yellow fever vectors. Should the travellers not be quarantined, they may be required to report any feverish or other symptoms to the competent authority and be placed under surveillance.
To be completed and submitted to the competent authorities by the masters of ships arriving from foreign ports.
Submitted at the port of ................................................................. Date .....................................
Name of ship or inland navigation vessel ....................................................... Registration/IMO
No .............................. arriving from ................................. sailing to ..........................................
(Nationality) (Flag of vessel) .................................. Master’s name ...........................................
Gross tonnage (ship) .....................................
Tonnage (inland navigation vessel) .........................................
Valid Sanitation Control Exemption/Control Certificate carried on board? yes ............ no ............ Issued at ................... date .....................
Re-inspection required? yes ....... no .......
Has ship/vessel visited an affected area identified by the World Health Organization? yes ..... no .....
Port and date of visit ........................................................
List ports of call from commencement of voyage with dates of departure, or within past thirty days, whichever is shorter:
.......................................................................................................................................................
Upon request of the competent authority at the port of arrival, list crew members, passengers or other persons who have joined ship/vessel since international voyage began or within past thirty days, whichever is shorter, including all ports/countries visited in this period (add additional names to the attached schedule):
(1) Name ........................................ joined from:
(1) ........................................... (2) ...........................................(3) ...........................................
(2) Name ........................................ joined from:
(1) ........................................... (2) ...........................................(3) ...........................................
(3) Name ........................................ joined from:
(1) ........................................... (2) ...........................................(3) ...........................................
Number of crew members on board ......................
Number of passengers on board ..............................
Health questions
(1) Has any person died on board during the voyage otherwise than as a result of accident? yes .... no .....
If yes, state particulars in attached schedule. Total no. of deaths ..........
(2) Is there on board or has there been during the international voyage any case of disease which you suspect to be of an infectious nature? yes ........ no ........ If yes, state particulars in attached schedule.
(3) Has the total number of ill passengers during the voyage been greater than normal/expected? yes .... no .....
How many ill persons? ..........
(4) Is there any ill person on board now? yes ........ no ........ If yes, state particulars in attached schedule.
(5) Was a medical practitioner consulted? yes ....... no ...... If yes, state particulars of medical treatment or advice provided in attached schedule.
(6) Are you aware of any condition on board which may lead to infection or spread of disease? yes ........ no ........
If yes, state particulars in attached schedule.
(7) Has any sanitary measure (e.g. quarantine, isolation, disinfection or decontamination) been applied on board? yes ....... no ......
If yes, specify type, place and date.
(8) Have any stowaways been found on board? yes ....... no ...... If yes, where did they join the ship (if known)? .........................................
(9) Is there a sick animal or pet on board? yes ......... no ........
Note: In the absence of a surgeon, the master should regard the following symptoms as grounds for suspecting the existence of a disease of an infectious nature:
(a) fever, persisting for several days or accompanied by (i) prostration; (ii) decreased consciousness; (iii) glandular swelling; (iv) jaundice; (v) cough or shortness of breath; (vi) unusual bleeding; or (vii) paralysis.
(b) with or without fever: (i) any acute skin rash or eruption; (ii) severe vomiting (other than sea sickness); (iii) severe diarrhoea; or (iv) recurrent convulsions.
I hereby declare that the particulars and answers to the questions given in this Declaration of Health (including the schedule) are true and correct to the best of my knowledge and belief.
Signed
..............................................
Master
Countersigned
..............................................
Ship’s Surgeon (if carried)
Date .................................................................
| Name | Class or rating | Age | Sex | Nationality | Port, date joined ship/vessel | Nature of illness | Date of onset of symptoms | Reported to a port medical officer? | Disposal of case* | Drugs medicines or other treatment given to patient | Comments | |
* State: (1) whether the person recovered, is still ill or died; and (2) whether the person is still on board, was evacuated (including the name of the port or airport), or was buried at sea.
THIS DOCUMENT IS PART OF THE AIRCRAFT GENERAL DECLARATION, PROMULGATED BY THE INTERNATIONAL CIVIL AVIATION ORGANIZATION
Declaration of Health
Name and seat number or function of persons on board with illnesses other than airsickness or the effects of accidents, who may be suffering from a communicable disease (a fever - temperature 38°C/100 °F or greater - associated with one or more of the following signs or symptoms, e.g. appearing obviously unwell; persistent coughing; impaired breathing; persistent diarrhoea; persistent vomiting; skin rash; bruising or bleeding without previous injury; or confusion of recent onset, increases the likelihood that the person is suffering a communicable disease) as well as such cases of illness disembarked during a previous stop ...
.......................................................................................................................................................
Details of each disinsecting or sanitary treatment (place, date, time, method) during the flight. If no disinsecting has been carried out during the flight, give details of most recent disinsecting ...................................................................................................................................
.......................................................................................................................................................
Signature, if required, with time and date ........................................................
Crew member concerned
1. A Nemzetközi Egészségügyi Rendszabályok (a továbbiakban: NER vagy Rendszabályok) alkalmazásában:
érintett: az a személy, poggyász, rakomány, konténer, szállítóeszköz, áru, postacsomag vagy emberi maradványok, akik/amelyek fertőzöttek vagy szennyezettek, vagy akik/amelyek fertőzés vagy szennyezés forrását hordozzák, és ezáltal közegészségügyi-járványügyi kockázatot jelentenek;
érintett terület: az a földrajzi hely, amelynek vonatkozásában az EVSZ kifejezetten ajánlja egészségügyi intézkedések megtételét e Rendszabályok értelmében;
légi jármű: nemzetközi utazást végrehajtó légi jármű;
légi kikötő: azok a repülőterek, ahova nemzetközi járatok érkeznek, illetve ahonnan indulnak;
érkezés a közlekedési eszköz tekintetében:
a) tengerjáró hajók esetében behajózás a kikötő egy meghatározott részébe vagy lehorgonyzás a kikötő egy meghatározott részében;
b) légi jármű esetében megérkezés a légi kikötőbe;
c) nemzetközi utazást végrehajtó belvízi vízi jármű esetében megérkezés egy belépési helyhez;
d) vonat vagy közúti jármű esetében megérkezés egy belépési helyhez;
poggyász: az utas személyes holmija;
rakomány: szállítóeszközön vagy szállítótartályban szállított áruk;
hatáskörrel rendelkező hatóság: a Rendszabály előírásai szerinti egészségügyi intézkedések megtételére és alkalmazására illetékes hatóság;
szállítótartály (konténer): az a szállító berendezés, amely
a) állandó jellegű és ebből eredően kellő szilárdságú ahhoz, hogy ismételten felhasználható legyen;
b) kifejezetten abból a célból készült, hogy az áruknak egy vagy több szállítási mód igénybevételével történő, átrakás okozta megszakítástól mentes fuvarozását megkönnyítse;
c) úgy készült, hogy könnyen kezelhető legyen, különösen egyik szállítási módról a másikra való átrakása során; és
d) úgy készült, hogy könnyen megrakható és üríthető legyen;
szállítótartály-rakodási terület: a nemzetközi forgalomban részt vevő szállítótartályok be- és kirakodására fenntartott hely vagy létesítmény;
szennyezés: fertőző vagy toxikus ágens vagy anyag jelenléte az ember vagy az állat testfelületén, fogyasztás céljára készített termékben vagy terméken, vagy bármely más élettelen tárgyon, ideértve a szállítóeszközöket is, amely közegészségügyi-járványügyi kockázatot jelenthet;
szállítóeszköz: nemzetközi utazást végrehajtó légi szállítóeszköz, vízi jármű, vonat, közúti jármű vagy egyéb közlekedési eszköz;
szállítóeszköz üzemben tartója: a szállítóeszközért felelős természetes vagy jogi személy, vagy annak megbízottja;
személyzet: a szállítóeszköz fedélzetén tartózkodó azon személyek, akik nem tartoznak az utasok közé;
dekontaminálás: az az eljárás, amelynek során egészségügyi intézkedéseket hajtanak végre annak érdekében, hogy eltávolítsanak az ember vagy az állat testfelületén, fogyasztás céljára készített termékben vagy terméken, vagy bármely más élettelen tárgyon, ideértve a szállítóeszközöket is, található fertőző vagy toxikus ágenst vagy anyagot, amely közegészségügyi-járványügyi kockázatot jelenthet;
indulás: a személyek, poggyász, rakomány, szállítóeszközök vagy áruk tekintetében egy terület elhagyása;
rágcsálómentesítés: az az eljárás, amelynek során egészségügyi intézkedéseket hajtanak végre a belépési helyen a poggyászban, rakományban, szállítótartályokban, szállítóeszközökben, létesítményekben, árukban és postacsomagokban található, emberi betegséget terjesztő rágcsálók/patkányok számának veszélyt nem okozó szinten tartására vagy kiirtására;
főigazgató: az Egészségügyi Világszervezet főigazgatója;
betegség: megbetegedés vagy kóros állapot, függetlenül annak eredetétől vagy forrásától, amely jelentős ártalmat okoz vagy okozhat az emberek számára;
fertőtlenítés: az az eljárás, amelynek során egészségügyi intézkedéseket hajtanak végre annak érdekében, hogy az emberi vagy állati testfelületen, vagy a poggyász, rakomány, szállítótartály, szállítóeszköz, áru, illetve postai csomag belsejében vagy annak felületén található kórokozókat elpusztítsák vagy fertőzőképességüket megszüntessék vegyi vagy fizikai behatás révén;
rovarmentesítés: az az eljárás, amelynek során egészségügyi intézkedéseket hajtanak végre a beléptetési helyen a poggyászban, rakományban, szállítótartályokban, szállítóeszközökben, létesítményekben, árukban és postacsomagokban található, emberi betegséget terjesztő rovarok számának veszélyt nem okozó szinten tartására vagy kiirtására;
esemény: betegség kialakulása vagy olyan történés, amely a betegség kialakulásának lehetőségét teremti meg;
szabad közlekedési engedély: hajónak adott engedély arra, hogy befusson a kikötőbe, behajózzon vagy kihajózzon, rakományt vagy készleteket rakodjon ki vagy be; a légi járműnek adott engedély arra, hogy leszállás után beszállítást és kiszállítást végezzen, rakományt vagy készleteket rakodjon ki vagy be; és a szárazföldi szállító járműnek adott engedély arra, hogy érkezéskor beszállítást vagy kiszállítást végezzen, rakományt vagy készleteket rakodjon ki vagy be;
áruk: materiális termékek, ideértve az állatokat és növényeket is, amelyeket nemzetközi utazás során szállítanak, ideértve a szállítóeszköz fedélzetén történő fogyasztásra szánt árukat is;
szárazföldi átkelőhely: szárazföldi belépési hely a Részes Államba, ideértve a közúti járművek és vonatok által használt átkelőhelyeket is;
földi szállító jármű: szárazföldi szállításra szolgáló, nemzetközi utazást végrehajtó gépi meghajtású szállítóeszköz, ideértve a vonatot, buszt, teherautót és személygépkocsit;
egészségügyi intézkedés: azok az eljárások, amelyeket betegség vagy szennyezés terjedésének megelőzése céljából alkalmaznak; az egészségügyi intézkedésbe nem tartoznak bele az igazgatásrendészeti vagy biztonsági intézkedések;
beteg személy: az az egyén, aki olyan testi bajban szenved vagy olyan testi baj hatásait viseli magán, amely közegészségügyi-járványügyi kockázatot jelenthet;
fertőzés: kórokozó behatolása és fejlődése vagy szaporodása az ember és az állat szervezetében, amely közegészségügyi-járványügyi kockázatot jelenthet;
ellenőrzés: a területek, poggyász, szállítótartályok, szállítóeszközök, létesítmények, áruk vagy postacsomagok, ideértve a vonatkozó adatokat és dokumentációt is, hatáskörrel rendelkező hatóság általi vagy annak felügyelete mellett történő átvizsgálása annak a megállapítása céljából, hogy fennáll-e közegészségügyi-járványügyi kockázat;
nemzetközi forgalom: személyek, poggyász, rakomány, szállítótartályok, szállítóeszközök, áruk vagy postacsomagok nemzetközi határt átlépő mozgása, ideértve a nemzetközi kereskedelmet is;
nemzetközi utazás:
a) szállítóeszköz tekintetében egynél több állam felségterületére való belépési helyek közötti utazás, vagy ugyanazon állam felségterületére vagy területeire való belépési helyek közötti utazás, ha a szállítóeszköz az utazása során kapcsolatba kerül valamely más állam felségterületével, de kizárólag e kapcsolatok vonatkozásában;
b) utasok tekintetében az az utazás, amelybe beletartozik az attól az államtól eltérő állam felségterületére történő belépés, amely államban az utas megkezdte az utazást;
tolakodó: közvetlen vagy bizalmas érintkezés vagy kikérdezés révén kényelmetlenséget előidéző;
invazív: a bőrön áthatoló szúrás vagy vágás, vagy egy műszer vagy idegen anyag bevezetése a testbe, vagy egy testüreg vizsgálata. A Rendszabályok alkalmazásában a fül, orr és a száj orvosi vizsgálata, hőmérsékletmérés fülben, szájban vagy hónaljban, vagy termikus képalkotással; orvosi vizsgálat; auszkultáció (hallgatózás); külsőleges palpáció (tapintásos vizsgálat); retinoszkópia (szemtükrözés); vizelet-, széklet- vagy nyálminta testen kívüli vétele, külsőleges vérnyomásmérés és elektrokardiográfia nem-invazív eljárásoknak tekintendők;
elkülönítés: a beteg vagy szennyezett személyek, vagy érintett poggyász, szállítótartályok, szállítóeszközök, áruk vagy postacsomagok elválasztása a többitől a fertőzés vagy szennyezés terjedésének megakadályozására alkalmas módon;
orvosi vizsgálat: előzetes státuszfelmérés, amelyet engedéllyel rendelkező egészségügyi dolgozó vagy a hatáskörrel rendelkező hatóság közvetlen felügyelete alatt álló személy végez el az egyén egészségi állapotának meghatározása és az esetleg másokra jelentett közegészségügyi-járványügyi veszély megállapítása céljából. Ez magában foglalhatja az egészségügyi dokumentumok átvizsgálását és a fizikális vizsgálatot is, amennyiben az adott eset körülményei azt indokolják;
nemzeti NER tájékoztatási központ: a Részes Államok által kijelölt nemzeti központ, amely folyamatosan hozzáférhető a Rendszabályok előírásai szerinti EVSZ NER kapcsolattartó központokkal való kommunikáció céljából;
Szervezet vagy EVSZ: az Egészségügyi Világszervezet;
állandó lakóhely: az érintett Részes Állam belső jogszabályaiban meghatározott jelentéssel bír;
személyes adatok: azonosított vagy azonosítható természetes személyre vonatkozó összes információ;
belépési hely: utasok, poggyász, rakomány, szállítótartályok, szállítóeszközök, áruk és postacsomagok nemzetközi belépésére vagy kilépésére szolgáló átjáró, továbbá az ezek számára belépéskor vagy kilépéskor szolgáltatásokat biztosító szervek és területek;
kikötő: tengeri kikötő vagy belvízi víztesten elhelyezkedő kikötő, ahova, illetve ahonnan nemzetközi utazást végrehajtó hajók érkeznek, illetve indulnak;
postacsomag: címzéssel ellátott küldemény vagy csomag, amelyet nemzetközi viszonylatban postai vagy futárszolgálat szállít;
nemzetközi horderejű közegészségügyi-járványügyi szükséghelyzet: az a rendkívüli esemény, amelyet a Rendszabályok előírásaival összhangban akként minősítenek, hogy az
(i) közegészségügyi-járványügyi kockázatot jelent más államokra nézve a betegség nemzetközi terjedése révén, és
(ii) esetlegesen összehangolt nemzetközi reagálást tesz szükségessé;
közegészségügyi-járványügyi megfigyelés: az utas egészségi állapotának figyelemmel kísérése hosszabb távon a betegség átadása kockázatának meghatározása céljából;
közegészségügyi-járványügyi kockázat: az emberi populációk egészségére károsan ható esemény valószínűsége, különös hangsúllyal azon az eseményen, amely nemzetközileg terjedhet, vagy amely súlyos és közvetlen veszélyt okozhat;
járványügyi zárlat (karantén): gyanús, de nem beteg személyek tevékenységeinek korlátozása és/vagy e személyek, vagy gyanús poggyász, szállítótartályok, szállítóeszközök vagy áruk elkülönítése másoktól a fertőzés vagy szennyezés lehetséges terjedésének megakadályozása céljából;
ajánlás és ajánlott: a Rendszabályok értelmében kiadott ideiglenes vagy állandó hatályú ajánlások;
kórokozó-hordozó (rezervoár): az az állat, növény vagy anyag, amelyben egy adott fertőző kórokozó általában él, és amelynek jelenléte közegészségügyi-járványügyi kockázatot jelent;
közúti jármű: földi szállító jármű a vonat kivételével;
tudományos bizonyíték: a tudomány megalapozott és elfogadott módszereire alapuló bizonyítási szintet képviselő információ;
tudományos elvek: a természetnek a tudományos módszerek révén ismertté vált, elfogadott alaptörvényei és alapvető tényei;
hajó: nemzetközi utazást végrehajtó, tengeren vagy belvízen közlekedő vízi jármű;
állandó hatályú ajánlás: egy meghatározott, folyamatosan fennálló közegészségügyi-járványügyi kockázat tekintetében az EVSZ által a Rendszabályok 16. cikke értelmében kiadott, nem kötelező erejű javaslat olyan rutinszerűen vagy időszakosan alkalmazandó célirányos egészségügyi intézkedésekre vonatkozóan, amelyek a betegség nemzetközi terjedésének a megelőzése vagy csökkentése, valamint a nemzetközi forgalom zavarásának minimálisra csökkentése érdekében szükségesek;
felügyelet (surveillance): adatok közegészségügyi-járványügyi célból történő rendszeres, folyamatos gyűjtése, összehasonlítása és elemzése, valamint a közegészségügyi-járványügyi információk kellő időben történő terjesztése értékelés és szükség szerinti közegészségügyi-járványügyi reagálás céljából;
gyanús: az a személy, poggyász, rakomány, szállítótartály/konténer, szállítóeszköz, áruk vagy postacsomag, aki/amely a Részes Állam megítélése szerint közegészségügyi-járványügyi kockázatnak volt vagy lehetett kitéve, és aki/amely a betegség terjedésének lehetséges forrása lehet;
ideiglenes hatályú ajánlás: nemzetközi horderejű közegészségügyi-járványügyi szükséghelyzetre való reagálásként az EVSZ által a Rendszabályok 15. cikke alapján kiadott, nem kötelező erejű javaslat korlátozott idejű és kockázat-specifikus alkalmazásra, a betegség nemzetközi terjedésének a megelőzése vagy csökkentése, valamint a nemzetközi forgalom zavarásának minimálisra csökkentése érdekében;
ideiglenes lakóhely: az érintett Részes Állam belső jogszabályaiban meghatározott jelentéssel bír;
utas: nemzetközi utazást végrehajtó természetes személy;
kórokozó-terjesztő (vektor): az a rovar vagy más állat, amely közegészségügyi-járványügyi kockázatot jelentő fertőző kórokozót hordoz;
megerősítés: a Részes Állam által az EVSZ számára nyújtott tájékoztatás, amely megerősíti a szóban forgó Részes Állam felségterületén vagy területein kialakult esemény státusát;
EVSZ NER kapcsolattartó központ: az EVSZ-nek az a szervezeti egysége, amely folyamatosan hozzáférhető a nemzeti NER tájékoztatási központokkal való kommunikáció céljából.
2. Eltérő rendelkezés vagy a szövegösszefüggés ellenkező meghatározása hiányában a Rendszabályokra történő hivatkozás magába foglalja a mellékleteket is.
A Rendszabályok célja és alkalmazási köre kiterjed a betegségek nemzetközi terjedésének megakadályozására, az ellene való védekezésre, a terjedés leküzdésére és a közegészségügyi-járványügyi reagálásra a közegészségügyi-járványügyi kockázatokkal arányos és azokra korlátozódó, a nemzetközi forgalmat és kereskedelmet szükségtelenül nem zavaró módon.